What is nuclear fusion? Simply, fusion is when two elementary particles collide and combine or fuse to make -- even temporarily -- a new particle. In the graphic below, a form of the hydrogen nucleus -- Tritium -- collides with another form of the hydrogen nucleus -- Deuterium -- to form a new particle. (Deuterium and Tritium are isotopes of hydrogen -- a form of water.) The fused particle is not stable, and quickly decays via the split decay mode into a neutron -- moving very fast, i.e., "hot" -- and a helium neucleus -- also know as an "alpha" particle and energy.
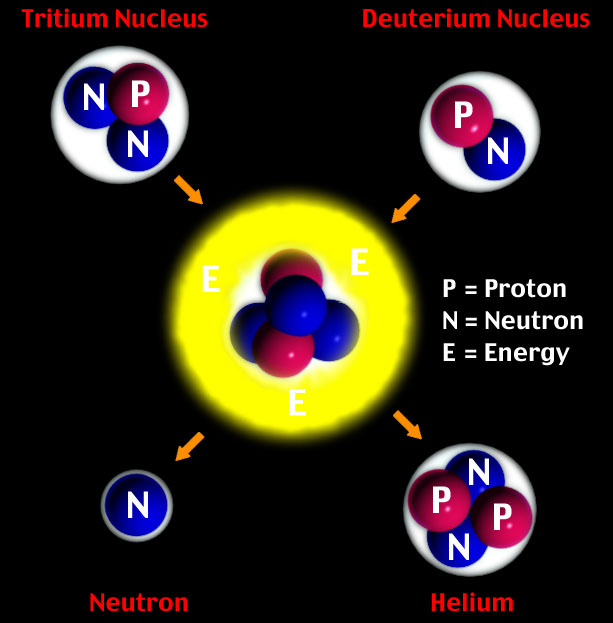
What is nuclear fusion? Fission is the opposite of fusion. A heavy elementary particle, splits into two or more lighter particles. (Note: during the fusion process, fission does occur in the second half of the process. When the large, unstable particle in the graphic above splits into the the neutron and the helium nucleus, it is a form of fission.) Normally, people associate the fission process with heavy elements like uranium or plutonium splitting into lighter elements.
The radioactive by-products from fission are undeniably bad. They last for tens of thousands of years and can cause untold problems, the worst of which is through terrosists using the waste products for weapons. The fast neutron from fusion can potentially interact with metals a cause them to become radioactive. This process can be minimized by have neutron shielding between the fusion burn and any metals in the reactor. However, the radioactive metals produced -- and any ash from the fusion burn itself -- is a low-level radioactivity that is not considered dangerous, with half-lives in the tens of years. There is no comparison -- radioactive waste from fission is very dangerous, radioactive waste from fusion is not very dangerous.
When fission or fusion occurs, the process of converting elementary particles from one type of particle to another is not "perfect." If you were to measure the mass of the particles after fusion or fission, the total mass would be less than before. For example, typical fusion reactions and the energy released are given in the table below:
| D + D | He3 + n |
3.27 MeV
|
| D + D | T + p |
4.04 MeV
|
| T + D | He4 + n |
17.26 MeV
|
| He3 + D | He4 + p |
18.34 MeV
|
| Li6 + D | 2He4 |
22.4 MeV
|
| Li7 + p | 2He4 |
17.3 MeV
|
| He3 + He3 | 2p + He4 |
12.86 MeV
|
It is hard for most people to understand where this energy comes from, and how much it is. Most people have heard of Einstein's famous equation:
E = mc2
When the fusion reactions listed above occur, a small amount of mass disappears. The small amount of mass is converted into a large amount of energy. How much is large? A typical chemical reaction in which hydrogen and oxygen combine to produce water, releases about 1 eV of energy. The fusion process typically releases 1,000,000 times as much energy. Historically, when people talk about the benefits of nuclear fusion, they refer to how much fuel must be burned to equal to each other.
| Process | Fuel | Amount |
| Fusion | Deuterium & Tritium | 1/2 ton pickup truck |
| Fission | Uranium Oxide | 1.5 train-car loads |
| Solar | Sun | 5000 acres of solar collectors plus energy storage for night and cloudy days |
| Burning Oil | Oil | 11 super tankers |
| Burning Coal | Coal | 250 train loads |
This does not even talk about pollution comparisons. I will put up another page on the overall benefits of nuclear fusion, but suffice to say, the relative amount of pollution for nuclear fusion is dramatically less than the other processes.
Nuclear fusion takes water, burns it, releases helium (and a small amount of radiation in the form of neutrons), and converts the missing mass into massive amounts of energy.